The Persistence of Non-Vitamin K Antagonist Oral Anticoagulants in Korean Patients with Non-Valvular Atrial Fibrillation
Article information
Abstract
Background and Objectives
Non-vitamin K antagonist oral anticoagulants (NOACs) are increasingly used for stroke prevention in patients with non-valvular atrial fibrillation (NVAF), showing better efficacy and safety than warfarin. However, the rates or reasons for discontinuation of NOACs in clinical practice have not been clarified. The aim of this study was to compare 3 NOACs (apixaban, rivaroxaban, and dabigatran) with warfarin in terms of medication persistence.
Subjects and Methods
We retrospectively evaluated 1,527 patients with NVAF who had recently started taking NOACs between January 2012 and September 2015 (294 apixaban, 748 rivaroxaban, and 485 dabigatran) and compared them with 363 patients with NVAF who started taking warfarin between January 2012 and December 2013 at the Samsung Medical Center.
Results
The mean follow-up duration was 532 days. The discontinuation rates were higher in the 3 NOAC groups than in the warfarin group within the first year. The major causes of discontinuation were maintenance of sinus rhythm; adverse events, including all bleeding and gastrointestinal symptoms; and patients demand. The adverse event rate was lower in the warfarin group than in the 3 NOAC groups. No significant differences in thromboembolic and major bleeding events were found between the 3 NOAC groups and the warfarin group.
Conclusion
In a single-center study, NOACs showed lower medication persistence and higher adverse event rates than warfarin during the first year.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice. AF is a global health-care problem with an increasing prevalence and incidence [1]. Patients with AF are at increased risk of death, heart failure, hospitalization, and thromboembolic events [2]. AF-associated strokes are often severe, resulting in disability or death [3]. Thus, an oral anticoagulation therapy such as vitamin K-antagonist anticoagulants (VKAs) is important for the prevention of stroke in patients at risk of AF.
Recently, several non-vitamin K-antagonist oral anticoagulants (NOACs) have been introduced. Randomized clinical trials (RCTs) showed that NOACs were superior to VKAs in efficacy and safety of preventing stroke and systemic embolisms in patients with non-valvular AF (NVAF) [4–6]. Current AF guidelines recommend risk stratification and use of NOACs [7].
NOACs provide lifestyle advantages, and efficacy and safety in stroke prevention. The use of NOACs is gradually increasing worldwide. The aim of this study was to evaluate the medication persistence of NOACs in comparison with that of warfarin.
Subjects and Methods
Study population and study design
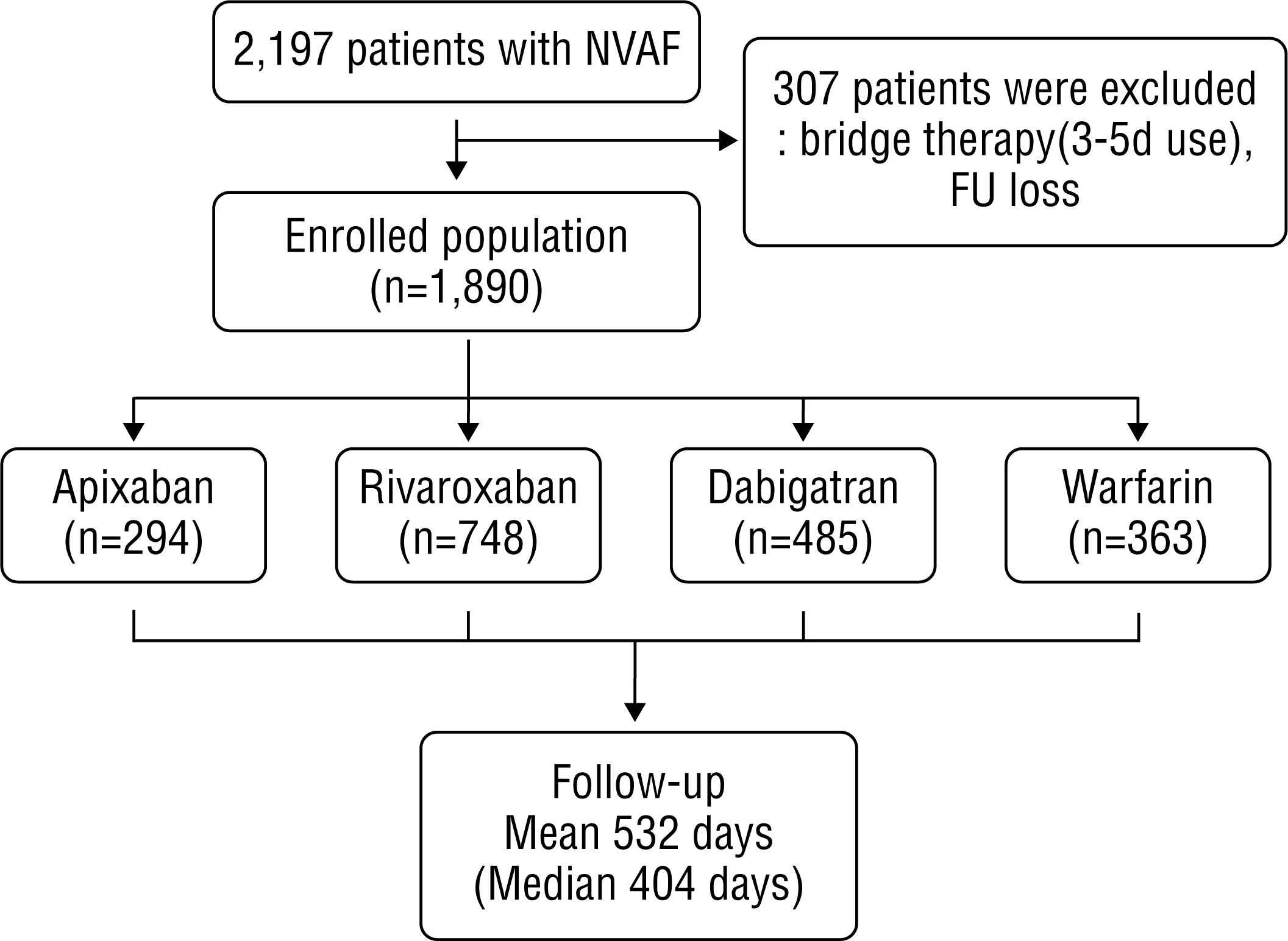
This is a retrospective study of patients with NVAF who were indicated for AF treatment. We included 1,527 patients with NVAF who had newly started NOAC therapy between January 2012 and September 2015 (294 apixaban cases between March 2014 and September 2015, 748 rivaroxaban cases between July 2012 and September 2015, and 485 dabigatran cases between January 2012 and September 2015) and 363 patients with NVAF who started warfarin therapy between January 2012 and December 2013 at the Samsung Medical Center (Figure 1).
Clinical data were collected from the patients' medical records to identify patients to whom NOACs or warfarin was prescribed, and those diagnosed with AF. We excluded patients with valvular heart disease, such as patients with moderate or severe mitral stenosis, mild rheumatic mitral stenosis, or a history of valvular surgery.
Clinical characteristics and definition of variables
Information on baseline characteristics such as patient age, sex, risk factor, and underlying disease were collected from medical records and laboratory data. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, a diastolic blood pressure of ≥90 mmHg, or a history of treatment for hypertension.
Diabetes mellitus (DM) previously diagnosed by a physician was defined as treatment with hypoglycemic agents or indicated by poor glycemic control (defined as a glycohemoglobin A1c level of ≥6.5%).
Heart failure was defined in accordance with the American College of Cardiology/American Heart Association criteria. Creatinine clearance was measured by using the Cockcroft-Gault formula. Coronary artery disease was defined based on positive stress test results, coronary angiograms demonstrating at least 70% stenosis, coronary spastic angina detected in an ergonovine provocation test or a history of prior myocardial infarction or revascularization procedures.
Non-persistence is defined as not taking prescribed medications, which is different from non-adherence, which is considered when doses are missed, extra doses are taken, or doses are taken in the wrong quantity or at the wrong time [8]. The terms non-persistence and discontinuation are used interchangeably in this report.
Bleeding includes gastrointestinal bleeding (hematochezia and melena), hematuria, epistaxis, hemoptysis, hematemesis, gingival bleeding, hematoma, and intracranial bleeding. Major bleeding was defined as clinically overt bleeding accompanied by a decrease in hemoglobin level of at least 2 g/dL or transfusion of at least 2 units of packed red cells, occurring at a critical site, or resulting in death according to the criteria of the International Society of Thrombosis and Hemostasis [9]. Minor bleeding was defined as all other clinically overt bleedings that did not meet the criteria for major bleeding and were persistent or recurrent, necessitating contact with the health system for treatment [9]. Thromboembolic events were defined as documented hospital admission due to acute ischemic stroke/transient ischemic attack (TIA), acute myocardial infarction, or other systemic embolisms.
The CHA2DS2-VASc score was used to estimate the risk of stroke. The CHA2DS2-VASc score takes into consideration congestive heart failure/left ventricular ejection fraction of <40%, hypertension, age of ≥75 years (double), DM, previous diagnosis of stroke/TIA or systemic embolism (double), vascular disease (history of myocardial infarction, peripheral artery), age of 65–75 years, and female sex. Previous bleeding was defined as major or clinically significant bleeding.
The HAS-BLED score was used to estimate the risk of bleeding. The HAS-BLED score takes into consideration hypertension (uncontrolled, >160 mmHg systolic), abnormal renal and liver functions (dialysis, transplant, Cr level of ≥2.26/ cirrhosis, bilirubin level twice the normal, liver enzyme thrice the normal), stroke, bleeding, labile international normalized ratio (INR), elderly (age >65 years), drugs (antiplatelet and nonsteroidal anti-inflammatory drug), or alcohol.
For apixaban, the standard dose is 5 mg twice daily and the reduced dose is 2.5 mg twice daily. For rivaroxaban, the standard dose is 20 mg once daily and the reduced dose is 15 or 10 mg once daily. For dabigatran, the standard dose is 150 mg twice daily and the reduced dose is 110 mg twice daily.
Follow-up
Information on drug cessation, major cardiovascular events, and minor adverse events were obtained at routine or additional visits at our hospital. The patients were followed up until the end of the follow-up period or until discontinuation of the drug, loss to follow-up, or death. The follow-up period was until September 2016. Information concerning deceased patients was obtained from medical records, family members, the patients' general practitioners, and the hospitals to which they had been admitted. In the patients who started warfarin, we also collected prothrombin time (PT)-INR data.
Outcomes
Drug cessation was defined as discontinuation of the prescribed drug and physician's mention of drug cessation in the medical record. All cases of drug cessation were reviewed from the medical records, and the reasons for cessation were also obtained. Temporary discontinuation for specific reasons such as surgery was not considered as drug cessation. Major cardiovascular events are defined as thromboembolism and major bleeding. The occurrence of thromboembolic and bleeding events was evaluated through the medical records reviewed by the 2 investigators.
Statistical analysis
Summary data are presented either as mean and standard deviation (SD) or as number of patients. Differences in baseline characteristics between NOACs and warfarin were tested with chi-square analysis for categorical variables and analysis of variance for continuous variables. The incidence rates of events were compared between the individual groups by using the chi-square test. The Kaplan-Meier curves for the outcomes of major cardiovascular events and discontinuation according to the NOACs were created by using the log-rank test. P values of <0.05 were considered significant. Data analyses were performed by using the SPSS statistical version 20 software (SPSS Inc., Chicago, Illinois).
Results
Patient characteristics
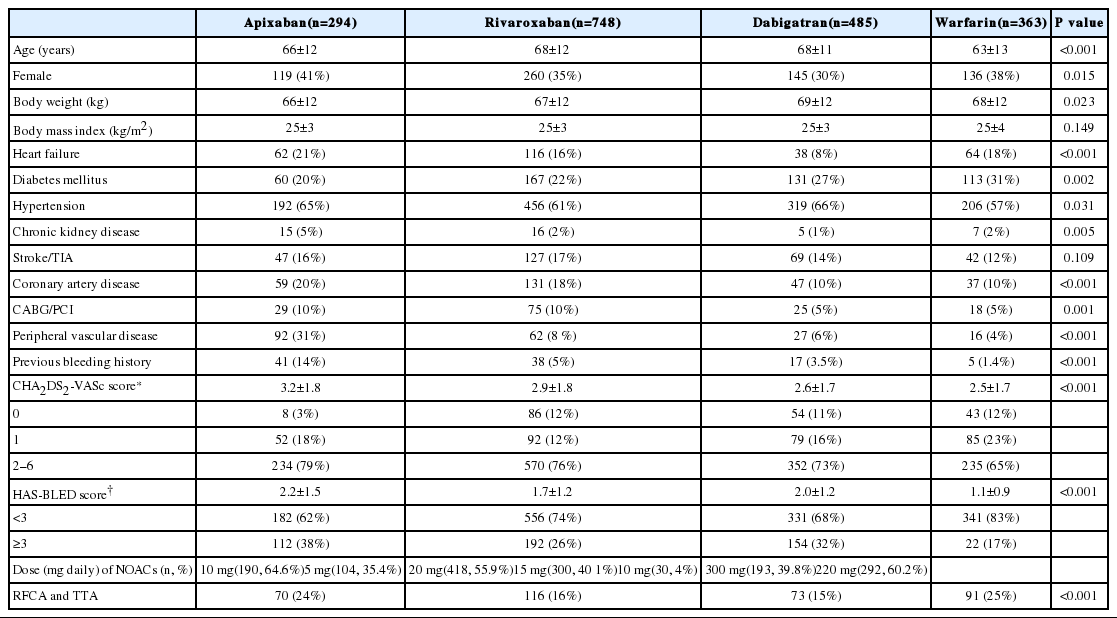
The baseline characteristics of the patients in the 3 NOAC groups and the warfarin group are shown in Table 1. Significant differences in age, sex, body weight, incidences of heart failure, hypertension, DM, chronic kidney disease, peripheral vascular disease, coronary artery disease, coronary artery bypass surgery/ percutaneous coronary intervention, and previous bleeding history were found among the groups. Significant differences in mean CHA2DS2-VASc and HAS-BLED scores were also found among the groups.
Discontinuation and persistence of NOACs and warfarin
The mean follow-up duration was 532 days (median, 404 days). The discontinuation rates and cause of cessation in the 3 NOAC groups and warfarin groups are shown in Table 2. The major causes of discontinuation were maintenance of sinus rhythm; adverse events, including all bleeding and gastrointestinal symptoms; and patient's request. Maintenance of sinus rhythm was the most common reason for discontinuing drugs in all the groups. Most cases of discontinuation on patient request were due to the costs of NOACs that are not covered by medical insurance. Gastrointestinal symptoms were more frequent than bleeding from adverse events in the dabigatran group. Various medical conditions included risk for interaction with concomitant medication, worsened renal function, cancer, and coronary artery intervention. The Kaplan-Meier curve for persistence of NOACs and warfarin are presented in Figure 2. The discontinuation rate in the warfarin group was lower than that in the NOAC groups. We excluded maintenance of sinus rhythm to calculate the persistence rate because many patients discontinued anticoagulation because of maintenance of sinus rhythm by operation or procedure such as radiofrequency ablation (RFCA) or total thoracoscopic ablation (TTA; Table 1). The Kaplan-Meier curves for persistence of NOACs and warfarin without maintenance of sinus rhythm are shown in Figure 3. The discontinuation rate was higher in all the NOAC groups than in the warfarin group. The incidence rate of adverse events in the warfarin group was lower than those in the 3 NOAC groups within the first year of treatment.

Kaplan-Meier curves for the persistence of NOACs and warfarin
NOACs, non-vitamin K antagonist oral anticoagulants
Major cardiovascular events
The warfarin group had lower mean CHA2DS2-VASc and HAS-BLED scores than the 3 NOAC groups. No significant differences in the incidence of thromboembolic and major bleeding events were found between the NOAC groups and the warfarin groups (Table 3). The warfarin group tended to have a higher incidence of major bleeding than the 3 NOAC groups, but the difference was not statistically significant.
Antithrombotic treatment after discontinuation
Antithrombotic treatments after discontinuation are shown in Table 4. The patients who discontinued NOACs mostly received another NOAC or an antiplatelet drug except for the apixaban group. Apixaban group tended not to receive any antithrombotic treatment. The patients who discontinued warfarin also received a NOAC or an antiplatelet drug.
Discussion
The persistence of NOAC and warfarin
This study showed the persistence and adverse events related to the administration of the 3 NOACs (apixaban, rivaroxaban, and dabigatran) in comparison with those related to warfarin administration in real-world practice. Only few reports have focused on this issue with respect to Korean patients with NVAF. Our data showed that both the NOAC and warfarin groups had low drug persistence. Approximately four-tenth of patients discontinued the drug in both groups. Medication persistence significantly differed between the NOACs groups and the warfarin group, but not during the total follow-up period in this study. Several aspects of our results should be considered. The follow-up duration in the warfarin group was 2 times longer than that in the NOAC group (435 vs. 943 days). The medication persistence in the NOAC group was lower than that in the warfarin group within the first year. The findings are shown in Figures 2 and 3. Maintenance of sinus rhythm, adverse events, and patient's request were the major causes of drug discontinuation. The proportion of maintenance of sinus rhythm was especially higher than those of other causes. Several patients had sinus conversion by ablation procedures such as RFCA or TTA in both the NOAC and warfarin groups. We evaluated the discontinuation rate without maintenance of sinus rhythm. The persistence in the NOAC group was still lower than that in the warfarin group within the first year after exception of maintenance of sinus rhythm (apixaban/rivaroxaban/dabigatran = 19.7%/21.5%/26.6% vs. warfarin = 17.4%).
The clinical trial of dabigatran in comparison with warfarin showed that the discontinuation rates for 110 mg of dabigatran, 150 mg of dabigatran, and warfarin were, respectively, 14.5%, 15.5%, and 10.2% at 1 year and 20.7%, 21.2%, and 16.6% at 2 years [4]. The clinical trial of apixaban in comparison with warfarin showed that fewer patients (25.3%) in the apixaban group than in the warfarin group (27.5%) discontinued a study drug before the end of the study [5]. The clinical trial of rivaroxaban in comparison with warfarin showed that the proportion of patients who discontinued their assigned drug was 23.7% in the rivaroxaban group and 22.2% in the warfarin group [6]. The discontinuation rates for the NOACs in the major clinical trials ranged from 21–25%. The major clinical trials [4–6] showed that the persistence rates in the dabigatran and rivaroxaban groups were lower than that in the warfarin group, and that the persistence rate in the apixaban group was similar to that of warfarin group. Our study finding on the persistence of anticoagulation drugs is consistent with the results of the RE-LY, ROCKET-AF, and ARISTOTLE trials. The reason of the better persistence of warfarin in this study is not clearly defined, but differences in tolerability may have contributed. The necessity of close monitoring by PT-INR in the warfarin group might be its better persistence. The incidence of adverse events was also lower in the warfarin group as was the persistence rate. The selection bias or lower CHA2DS2-VASc and HAS-BLED scores in the warfarin group could explain these findings. Several studies in clinical practice show that the warfarin group had better persistence than the NOAC group. A retrospective study by Tsuyoshi et al. [10] reported that the persistence in Japanese patients with NVAF who received NOACs was lower than that in patients who received warfarin at 12 months (70% vs. 82%) The study that compared among warfarin (n=9,969), apixaban (n=1,352), rivaroxaban (n=2,074), dabigatran (n=2,701), and aspirin (n=4,540) showed that the persistence rates of all the anticoagulant treatments were high in patients with NVAF after 2-year follow-up (82.9%) and better persistence of warfarin (85%) and apixaban (85.9%) than dabigatran (74.4%) or rivaroxaban (77.4%) in clinical practice [11]. These findings are similar to those of our study.
Reasons of cessation and Major complication
In this study, the major causes of discontinuation were the adverse events or patient's request. Patient's request in major clinical trials accounted for 8–10% of the total treated population. This finding was similar in our study. The adverse events were not severe, and the minor bleeding or gastrointestinal symptoms were the major reasons. Minor bleeding does not lead to severe major bleeding. Compared with the other NOAC groups and the warfarin group, the dabigatran group showed a higher proportion of patients with gastrointestinal symptoms than those with bleeding due to adverse events. The incidence rates of thromboembolic and major bleeding events were not significantly different between the NOAC groups and the warfarin group in clinical practice. These results were lower than those of the major clinical trials [4–6]. However, these findings should be explained cautiously because this study was retrospective.
Difference of the drug approval time
The drug approval times of NOACs differ in South Korea. Dabigatran was first approved, which was followed by the approval of rivaroxaban and then apixaban. At the time NOACs were approved, its safety and effectiveness in the clinical real world was not clarified. Concerns have been raised regarding the potential risk of serious adverse events induced by NOACs in comparison with warfarin. Therefore, the discontinuation rates of dabigatran might be higher than that of rivaroxaban or apixaban. As apixaban is the most recently approved drug, the number of patients that can be evaluated is smaller than that of other NOACs.
Persistence to NOAC treatment
The effectiveness and safety of NOACs were identified continuously by recent studies. The clinical outcomes of AF, such as mortality, stroke, and cardiovascular events, are strongly dependent on the quality of anticoagulation. Although NOACs were more convenient than warfarin as it does not require regular laboratory examination such as PT-INR and lifestyle advantage, it could decrease patient's attention to disease. The patient-physician relationship is important for better adherence. Physicians should provide sufficient information and explain the risk of discontinuation, and the necessity of consultation before drug discontinuation.
Limitations
This study has several limitations. This study was a retrospective observational study in a single center. We could not detect all minor events due to dependence on medical records. The results of this study might not reflect the situation in the average patient population in South Korea. The follow-up duration was relatively short. The starting points and follow-up period differed between the NOAC and warfarin groups because of the differences in drug-approved times.
Conclusion
In this study of AF patients, NOACs showed lower persistence rate than warfarin.
Disclosures
None
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
None